We're using mathematics to understand antimicrobial resistance (AMR) and develop new strategies to tackle bacterial infections. By carefully combining appropriate modelling approaches with experimental data, we are helping to accelerate the next generation of antibacterial treatments.
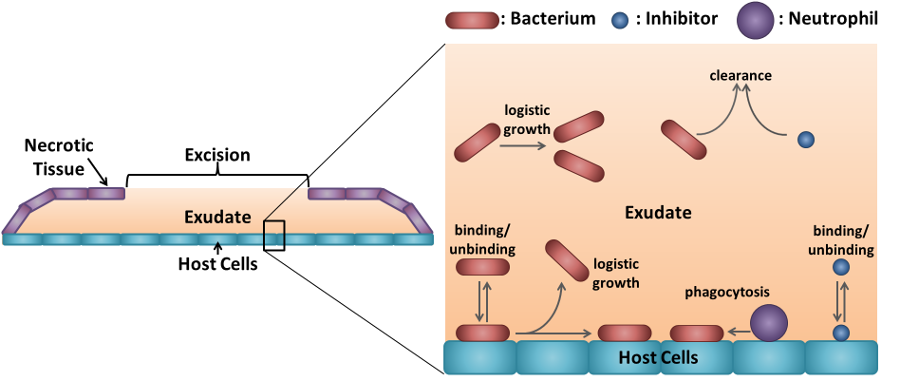 Diagram illustrating how anti-adhesion therapy combats bacterial colonisation of burn wounds. Credit: Image created by Dr. Paul A. Roberts
Diagram illustrating how anti-adhesion therapy combats bacterial colonisation of burn wounds. Credit: Image created by Dr. Paul A. Roberts
Growing antimicrobial resistance (AMR) threatens the effective prevention and treatment of a range of infections caused by bacteria, parasites, viruses and fungi. It jeapordises the success of major surgery and cancer chemotherapy. The World Health Organisation recognises it as an increasingly serious threat to global public health that requires action across all government sectors and society. Taking an interdisciplinary approach to tackling AMR will be crucial in ensuring this worldwide problem is solved as effectively as possible.
News
Curent research
We have active research collaborations with colleagues from the Healthcare Technologies Research Institute, Institute of Microbiology and Infection and the Schools of Biosciences and Chemistry as well as international researchers based at other institutions.
Our research primarily focuses on models of bacterial infections and novel approaches to treat these, either through new treatment types (e.g. anti-virulence drugs that prevent bacteria from deploying virulence mechanisms), by exploiting bacterial phenotypes (e.g. persister cells), or by targeting known resistance mechanisms. Some examples of our research include:
- preventing bacteria from causing infection using adhesion inhibitors that block the bacteria’s ability to bind to host tissue;
- investigating changes to bacterial cell morphology that enable the bacteria to evade antibiotic action, and crucially how to exploit these changes for therapeutic gain;
- examining the regulation of bacterial efflux pumps (a key mechanism behind antibiotic resistance) with a view to identifying suitable drug targets.
By using reliable mathematical models of bacterial infections, we are able to explore a huge range of possibilities that would not usually be feasible in the laboratory. In doing so, we help to refine and reduce the volume of experimental work required in the drug development process.
Our people
- Dr Sara Jabbari, Senior Lecturer in mathematical biology
- Emma Keen, Laboratory Technician
Selected publications