Have you heard of the seed revolution?
The fact that plants reproduce by making seeds is so basic that most of us don’t even stop to think about it, but seeds are crucial to us as a source of nutrition. Seeds represent the end-point of a highly complex and varied sexual reproductive process in plants, frequently produced within specialized edible lures (aka fruit) that plants will only make if they detect that seeds are developing inside of them. The vast majority of our agricultural crops are therefore the products of this specialized reproductive process, and with climate change and increasing pressure on our agricultural systems our future food security is dependent on improving our understanding of how plants do this.
Plants that make seeds are so commonplace today it is easy to assume that this has always been the case, but seeds are in fact an evolutionary (and revolutionary) innovation by one group of plant relative newcomers that proved so successful it has changed the face of life on Earth. Plants originally reproduced without seeds, instead dispersing single-celled spores much more similar to modern pollen (Figure 1). This switch from spores to seeds involved numerous complex changes in plant development- not just the seed itself, but also the invention of dedicated male and female haploid sexes, specialized reproductive structures (flowers and the carpel) and the process of pollination. How this revolution in plant reproduction was achieved remains one of the great unanswered questions of plant evolution, and answering this question can provide us with important insights into the mechanisms that control seed-based reproduction in modern plants.
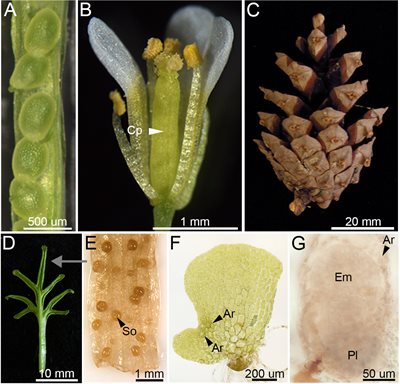
Figure 1. A quick comparison of seed-bearing and seedless plant reproduction:
The seeds of flowering plants (angiosperms) (A) contain a dormant plant embryo inside a protective coat together with a dedicated food supply (endosperm). Angiosperm seeds form inside the carpel (Cp) of the flower (B). Seed-bearing relatives of angiosperms, the gymnosperms, instead produce ‘naked’ seeds, for example between the scales of a pine cone (C). In place of seeds, ferns produce single-celled spores on the underside of their leaves (D, E), in special structures called sporangia or ‘sorii’ (So). Each spore germinates into a multi-celled photosynthetic ‘gametophyte’ (F), which eventually makes egg cells inside special reproductive organs called archegonia (Ar). Once fertilized inside the archegonium (G), this egg develops into an embryo (Em) on top of the gametophyte, which provides it with food through a placenta (Pl). This phase of vegetative growth present in seedless plant gametophyte development has been drastically reduced in seed-bearing plants, with the gametophyte now growing entirely inside the developing seed.

My research group is focused on investigating the evolution of seed-based reproductive systems at the level of the genetic circuitry that controls them. Survivors of the older, seedless plant families from which seed-based reproduction arose are still alive today, the most closely related to all living seed-bearing plants being the ferns. However, to-date astonishingly little is known about the genetic pathways that control development and reproduction in seedless plants. Using new experimental tools in these seedless plant groups (such as genetic modification of the fern Ceratopteris richardii1,2, Figure 2 and 3), we aim to compare genetic networks and gene functions between seedless and seed-bearing plant reproductive systems to identify what genetic changes took place during the origin of seed-bearing plants and how they have diversified since.
Figure 3. The model fern, Ceratopteris richardii, in full ‘flower’
Current Research projects
The lab is currently working on projects to compare the function of regulatory genes (transcription factors) found in both ferns and seed-bearing plants, where these genes have an important role in controlling reproduction in the model flowering plant Arabidopsis thaliana. Previously we found that the LEAFY floral regulator functions in vegetative development in ferns (Figure 3), suggesting that the genetic network controlling floral development first arose from a vegetative shoot.1 We are now focusing on the MADS-box genes, which in Arabidopsis act downstream of LEAFY to specify the identity of developing seeds and other floral organs.

Figure 3. Mapping LFY gene expression in C. richardii with a transgenic reporter. Fern fronds taken from plants of different ages (A, 20 days; B, 60 days; C, 124 days) showing blue staining wherever the gene CrLFY1 is being expressed. This gene is a master regulator of floral development- we didn’t expect to find this supposedly reproductive gene involved in fern leaf development! Taken from Plackett, Conway et al. 20181.
References
1. Plackett, Conway et al. (2018), eLIFE 7: 39625
2. Plackett et al. (2014), Plant Physiology 165: 3