
Microscopy Facility

The Microscopy Facility, mainly located at the Imaging suite, provides a wide portfolio of imaging platforms including Widefield and Confocal microscopes with fast detectors.
Most instruments are suitable for live imaging applications and this includes Selective Plane Illumination Microscopy (SPIM) system used with small animal models or spheroids. The creation of virtual slides through imaging of whole tissue mounts can be achieved with the AxioScanZ.1 slide scanner. Equipment specifications can be obtained from the following sections.
Microscopy Facility Team
Microscopy Facility Team
Microscopy Specialist
Dr Emma Faulkner
Emma is a Microscopy Specialist with expertise in advanced imaging techniques. She earned her PhD from the University of Birmingham in 2021, validating expansion microscopy for studying nuclear processes via DNA damage response proteins. She then worked as a postdoctoral fellow at the Department of Cardiovascular Sciences, using multi-dimensional imaging to analyse platelet aggregations. Now, she supports users at the Institute of Biomedical Research’s microscopy facility, assisting with imaging workflows and data acquisition.
Email: e.l.faulkner@bham.ac.uk
Microscopy Specialist Technician
Jacob Ballard
Jacob joined the Microscopy Facility in 2025. He was awarded a BSc (Hons) in Biochemistry from the University of Worcester in 2019 and has subsequently held research technician posts across a range of academic departments, including the Healthcare Technologies Institute. He has also worked within University Hospitals Birmingham NHS Trust, where he was responsible for the sectioning and staining of patient tissue specimens to support the diagnosis of cancer and other diseases. His technical expertise encompasses confocal microscopy, slide scanning, and a broad range of sample preparation methodologies.
Email: j.ballard.1@bham.ac.uk
Research Facilities Manager
Adriana Flores-Langarica
Email: A.FloresLangarica@bham.ac.uk
Microscopy Facility Academic Lead
Professor Steve Thomas
Email: s.thomas@bham.ac.uk
Training and Support
Training and Support
We provide training and support for users to independently use all our available equipment available in Stratocore. Currently, our training programmes are divided in two sections. Firstly, the CANVAS Module which can be completed at the user pace and once that is completed we deliver an in person training session at the equipment. If you require training you can request it in Stratocore. We deliver sessions regularly.
For our bespoke services, please reach out to us to discuss your project and how we can help you.
Access and Booking
Access and Booking
External users are encouraged to contact us via email in the first instance, however, all internal prospective users will need to request an account on the core facilities booking system Stratocore (unless you already have one). Please ensure you use your UoB email address to register your Stratocore account
As part of your account, you will need a valid finance/grant code. We need this to be able to charge you for your use of the equipment.
Your code will either be a project or a general ledger. Guidance on finance/grant codes is here:
Project Ledger. (Project Finance Number begins with a ‘1’ or ‘2’). Project Ledger codes require 3 components in the format:
Project Ledger
| Cost Centre Code | Task Number | Project Finance Number |
|---|---|---|
| C082 | 1.1 | 21991 |
General Ledger. (Project Finance Number begins with a ‘6’). General Ledgers require 2 components in the format:
| Cost Centre Code | Project Finance Number |
|---|---|
| C283 | 65371 |
If you are unsure about any of your Finance code, contact techubfinance@contacts.bham.ac.uk who will be able to assist you.
Account requests and flow cytometry training requests may be submitted simultaneously. GCP level users are required to submit a new project form through Stratocore before requesting flow cytometry training.
For any Stratocore or finance relation queries please contact techubfinance@contacts.bham.ac.uk
Equipment
Equipment
High Content Widefield and Fluorescence
Sartorius IncuCyte S3 Live-Cell Analysis System

The IncuCyte S3 system is ideal for long-term, automated live-cell analysis of up to 6 plates. The system resides inside an incubator which enables consistent maintenance of environmental controls at all times of the day and enables long-term experiments.
Images can be captured in HD phase and fluorescence (green and red) at 4x, 10x and 20x.
The IncuCyte software allows remote setup, data visualisation and data analysis. The basic version of the software provides capabilities for experiments such as cell counting, cell viability, cytotoxicity, cell cycle, immune cell, chemotaxis, whole well and dilution cloning. Additional experiments are available to purchase in agreement with the Facility.
Remote data analysis includes; (A) automatically acquire images over time, (B) use Incucyte® VesselView to view images of all locations in the vessel at once and quickly assess experimental results, plus zoom in on images of interest (C) automatically identify regions of interest via masks (D) generate presentation-ready timelapse graphs, and (E) view all 96- or 384-well kinetic trends at once with Incucyte® PlateGraph and export data to calculate EC50 or IC50 response values.
Features
Speed and Sensitivity In Mind
CMOS camera and efficient optics enable long-term live-cell imaging and analysis20 total core processors allowing for fast image processing and data analysis
Data Protection & Storage
Includes 27.3 TB of RAID hard drive data storage, which can be further extended with the 32.7 TB Incustore
LCD Control Panel
Monitor and perform basic status checks and operations from the front panel of the Incucyte S3 System controller unit
Software and Network Drives
IncuCyte 2022B Rev2 – On workstations in WXG.90 or available via remote download.
Zeiss Axio Scan .Z1 Slide Scanner
Axio Scan .Z1
The fully automated Zeiss Axio Scan .Z1 slide scanner creates high resolution virtual slides of whole tissue sections. Full tissue scanning with automatic or interactive detection of the selected area. The large capacity 100 slide magazine allows for scanning of large batches. Easy slide loading with dedicated imaging frames. Fully adaptable and programmable with synchronised external high speed excitation and internal emission filter wheels. Auto file saving to personalised storage in a secure server location.
Sample illumination and cameras
| Light Source | Camera | Pixel size and resolution | Function |
|---|---|---|---|
|
Bright field LED |
3CCD colour 2MP Hitachi 1200x1600 HV-F202SCL |
4.4 x 4.4 µm 0.22 µm per pixel*
|
Immunohistochemistry e.g. H&E, HRP, IHC |
|
HXP120 fluorescent white light |
Hamamatsu Orca Flash 4 -4MP 2048x204816bit sCMOS |
6.45 x 6.45 µm 0.32 µm / pixel*
|
Multichannel fluorescence
|
|
|
|
*20x magnification |
|
Optics
| Objective lens | Magnification / NA |
|---|---|
|
Fluar |
5x / 0.25 M27 (course focusing) |
|
Plan-Apochromatic |
10x / 0.45 M27 |
|
Plan-Apochromatic |
20x / 0.8 M27 |
|
Plan-Apochromatic |
40x/ 0.95 M27 |
Fluorescence filter sets
| Filter set | Excitation | Beam Splitter | Emission | Examples of fluorophore combinations |
|---|---|---|---|---|
|
Zeiss 49 HE |
365 |
FT385 |
445/50 |
Dapi / Hoescht / BV421/ Pacific Blue/ eFluor450 / Alexa 405 / Alexa 350 |
|
Zeiss 38 HE |
470/40 |
FT495 |
525/40 |
eGFP / YFP / Alexa 488 / FITC / pacific Green / Qdot525 (with ET380nm exciter) / Horizon BV500 / BV510 |
|
Zeiss 43 HE |
545/25 |
FT570HE |
635/70 |
Cy3 / Alexa 555/ Rhodamine / pacific Orange / Qdot605 (with ET380nm exciter) / tdTomato / Texas Red / eFLuor605nv / BV605 / Propidium Iodide / PE |
|
Zeiss 64 HE |
587/25 |
FT605HE |
647/70 |
mPlum / Alexa 594 / Alexa 610 / Texas Red / Qdot655 (with ET 380nm exciter) / BV650 / |
|
Chroma ET49006 |
620/60 |
ET700/75 |
700/75 |
Cy5 / Alexa 633 / 647 / 700 / Qdot705 (with ET380nm exciter) |
Slide acceptability and capacity
Single width slide; (max 4 per mounting frame) x 25 width 24-26mm, length 74-76mm, depth 0.8-1.3mm.
Double width slide; (max 2 per mounting frame) x 2 width 52mm, length 74-76mm, depth 0.8-1.3mm.
Quad width slide; (max 1 per frame) width 104mm, length 74-76mm, depth 0.8-1.3mm (optional extra).
Software
ZEN blue (2012) slide scan software has capability to drive whole tissue or multi-region tiling with Z-stacking plus specific profiles for TMA’s and cell spreads etc.
Wide Field Fluorescence
Leica DM6000B upright widefield epifluorescence and colour microscope

The Leica DM6000B is multimodal and equipped with twin Leica cameras for capture in 4 fluorescence channels and colour bright field for histological examination. Based on an upright DM6000B stand with fully motorised stage and filter turret. Applicable for slide analysis only.
Optics
| Objective lens | Magnification / NA / Immersion | Contrast Method |
|---|---|---|
|
HC Plan Apo |
10x / 0.45 / Dry |
Phase 1 |
|
HC Plan Apo |
20x / 0.7 / Dry |
Phase 3 |
|
Plan Apo |
40x / 1.25 / Oil |
|
|
Plan Apo |
100x / 1.4 / Oil |
Phase 3 |
Epifluorescence filter sets
| Filter cube | Excitation nm | Emission nm | Fluorophores |
|---|---|---|---|
|
A4 |
360 |
Blue 470 |
Dapi / Hoescht / Pacific Blue / eFluor450 / Alexa Fluor 405 |
|
L5 |
480 |
Green 527 |
eGFP / YFP / Alexa 488 / FITC / pacific Green |
|
N3 |
546 |
Red 600 |
Cy3 / Alexa 555/ Rhodamine / pacific Orange / tdTomato / Texas Red / Propidium Iodide / PE |
|
Y5 |
620 |
Far Red 700 |
Cy5 / Alexa 633 / 647 / APC |
Software and Network Drives
- Leica LASX core
- Desk top script connections to personal offline storage folders
Leica DMI6000B widefield epifluorescence and brightfield microscope with timelapse

Based in the Centre for Translational Inflammation Research (CTIR), the Leica DMI 6000B is equipped with a DFL350 FX camera for capture in 3 fluorescence channels and bright field. Based on a fully motorised inverted DMI 6000B stand and environmental chamber with CO2 for live cell imaging. A time-lapse module is available for migration studies. Applicable for imaging slides, cell dishes, multi-well plates and chamber slides.
Optics
| Objective lens | Magnification / NA / Immersion | Contrast Method |
|---|---|---|
|
HCX Pl Fluotar |
20x / 0.4 / Corr Dry |
Phase 1 |
|
HCX Pl Fluotar |
40x / 0.6 / Corr Dry |
Phase 2 |
|
HCX Pl Fluotar |
63x / 0.7 / Corr Dry |
Phase 2 |
| Filter cube | Excitation nm | Emission nm | Fluorophores |
|---|---|---|---|
|
A4 |
360 |
Blue 470 |
Dapi / Hoescht / Pacific Blue / eFluor450 / Alexa Fluor 405 |
|
L5 |
480 |
Green 527 |
eGFP / YFP / Alexa 488 / FITC / pacific Green |
|
N3 |
546 |
Red 600 |
Cy3 / Alexa 555/ Rhodamine / pacific Orange / tdTomato / Texas Red / Propidium Iodide / PE |
Software and Network Drives
- Leica LASX core
- Desk top script connections to personal offline storage folders
Zeiss Axio Observer widefield system with Apotome 2.0 system

Motorised Axio Observer geared toward fluorescence performance with LED Colibri.2 illumination system that offers high speed switching times to the order of less than 1 ms. The unique feature of the Colibri.2 design in comparison with all other LED systems currently available is its unmatched homogeneity. The light path up to the illuminator output is the same for each LED module independent of its installation position. Four channels are included at 365nm, 470nm, 565nm, and 590nm. The system is equipped with an Hamamatsu ORCA Flash 4.0 for high speed and high sensitivity.
In addition, the system is equipped with the Apotome.2 Confocal Structured Illumination device for creating optical sections with high contrast. Despite the magnification used, the ApoTome.2 automatically places the right grid in the beam path of the microscope. Reduction of unwanted background fluorescence increases with the grid frequency and the optical sections become thinner. Structures from outside of the focal plane are suppressed improving contrast and resolution of the optical section.
Laser Scanning Confocal
Zeiss LSM880 Confocal Microscope with Airyscan Fast

The LSM 880 scanner has high sensitivity and is equipped with a 32 element galanium arsenide phosphide (GaAsP) Quasar detector, plus 2 standard PMTs and 1 transmitted light T-PMT. Equipped with a tuneable AOTF and capable of imaging up to 6 channels in multitrack configuration. The AxioObserver.Z1 inverted stand, is equipped with an environmental chamber with CO2 for live cell imaging. Focus stabilisation is through software and hardware based mechanisms (Definite focus II). Interchangeable stage inserts for slides, chamber slides, 35mm dishes and multiwall plates. Image capture in 3D and 4D, plus Lambda scanning, and spectral un-mixing capability.
Airyscanning
An additional scan detector providing 1.7x isotropic increase in resolution from widefield ~120nm. The Airy Disk or Point spread function from every light emitting point is projected through zoom optics and scanned by a hexagonal array for 32 GaAsP detectors which then undergoes a process of pixel reassignment and subsequent processing. All dyes and fluorophores that are scanned by the conventional detectors can be Airy-scanned using specific emission filter sets in simultaneous or sequential acquisition modes.
Airyscanning in Fast mode
In fast mode the beam is shaped to scan not one but 4 lines of pixels in each scanning sweep, acquiring 4x faster than normal Airyscan. Only the central 16 elements of the array are utilised providing a 1.5 x increase in resolution. Excellent for fast imaging of live cell dynamics with increased resolution
Optics
| Objective lens | Magnification / NA / Immersion | Contrast Method |
|---|---|---|
|
C-ACHROMAT |
10x / 0.45 / Water |
|
|
LD LCI Plan-NEOFLUAR |
25x / 0.8 / Water or Oil |
DIC II |
|
Plan-ACHROMAT |
40x / 1.2 / Water |
DIC III |
|
Plan-APOCHROMAT |
63x / 1.2 / Water |
DIC III |
|
Plan-APOCHROMAT |
63x / 1.4 / Oil |
DICIII |
|
Plan-APOCHROMAT |
100x / 1.4 / Oil |
DIC III |
Epifluorescence Filter Sets
| Filter set | Ex BP nm | Em BP nm |
|---|---|---|
|
Fs49 wf |
365 |
445/50 |
|
Fs38 wf |
470/40 |
525/50 |
|
Fs43 wf |
545/25 |
635/70 |
Airy Scan Emission Filter Sets
|
BP420-445 |
+ |
BP465-505 |
|
BP420-480 |
+ |
BP495-550 |
|
BP495-550 |
+ |
LP525 |
|
BP495-535 |
+ |
LP555 |
|
BP495-550 |
+ |
LP570 |
| BP570-620 | + | LP645 |
Laser Lines
- Lasos 30mW Diode 405nm
- Lasos 25mW LGN3001 multiline Argon, 458, 488, 514nm
- Lasos 20mW (DPSS 561) 561nm
- Lasos 2mW HeNe 594nm
- Lasos 5mW HeNe 633nm
Experimental Modes
- Z stack
- Multi-positions
- Time lapse
- Tile scanning / image stitching of large samples
- Bleaching and FRET analysis
Software and Network Drives
- Zen 2.3 allows for multidimensional experiment design in 2D, 3D, and 4D
- PC, HPZ840 4TB 3 RAID Hard drives and 64GB of memory.
- Desk top script connections to personal offline storage folders
Leica STELLARIS 8
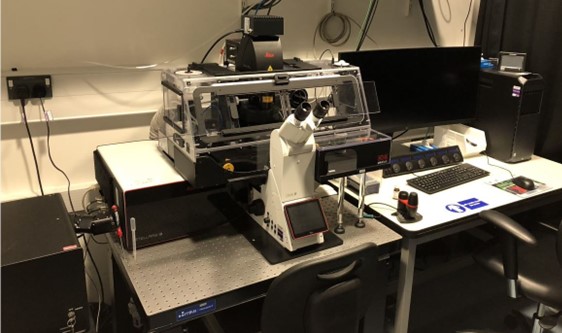
Leica STELLARIS 8 Digital LightSheet (DLS) unites in one place a confocal system and a light sheet microscope – a unique combination aimed to make your research more versatile. The exclusive vertical design of DLS, allows confocal and light sheet imaging to be combined in the same system. DLS also brings flexibility by the capacity to image different types of samples (model organisms, organoids, or cleared tissues) as well as the ability to perform fast volumetric imaging in near physiological conditions to study developmental processes. By combining excitation wavelengths in the far-red spectrum with the capacity to generate the light sheet with resonant scanner – which results in shorter pixel dwell times and, consequently less phototoxic effects – DLS and STELLARIS enable even gentler imaging.
There is the choice of more fluorescent dyes and even gentler imaging using excitation wavelengths in the far-red spectrum using the STELLARIS white light laser (WLL) as the excitation source to illuminate the sample with the possibility of using additional fixed lasers, significantly increasing the number of spectral possibilities for the imaging setup.
Obtain light sheet and confocal results with improved contrast and signal-to-noise ratio thanks to the LIGHTNING information extraction solution for all type of imaging data. LIGHTNING automatically considers the objectives that were used during acquisition to simulate the correct PSF of the system when processing the data. This way, it improves contrast and the signal-to-noise ratio and extends the image resolution for any channel by detecting the finest structures and details which are otherwise simply not visible.
High sensitivity, broad spectral coverage and a wide dynamic range are essential to take full advantage of a wide range of fluorescent probes, multi-label experiments and diverse applications. This system is equipped with Power HyD detector family designed for high performance in terms of spectral coverage, sensitivity and dynamic range. With their single-photon counting capabilities, Power HyD detectors also enable a unique set of fluorescence-lifetime-based imaging applications.
The vast majority of imaging experiments measure fluorescence intensity to study targets of interest, but fluorescence has another property that is always present, but not always measured: fluorescence lifetime. STELLARIS TauSense technology gives the potential to exploit fluorescence lifetime-based information to gain new functional insights, study more targets simultaneously, or simply improve signal-to-noise by removing unwanted signal.
For more information about specs, applications, training and access please contact one of the team members of the Microscopy Facility.
Specifications:
| Detectors | ||
|---|---|---|
| x3 Power HyD S | ||
| 1x Power HyD X | ||
| Filter Cubes (Microscope frame only) | ||
| LED405 | DsRed | GFP |
| Illumination | ||
| LED | Leica LED3 | |
| Lasers | 405nm Laser | |
| 488 nm Laser | ||
| 561nm Laser | ||
| 440-850 nm WLL | ||
| Optics (confocal) | ||
| HC PL APO Cs2 | 10x/0.40 air | |
| HC PL APO Cs2 | 20x/0.75 air | |
| HC PL APO oil Cs2 | 40x/1.30 oil | |
| HC PL APO oil Cs2 | 63x/1.40 oil | |
| Optics (Lightsheet illumination) | ||
| HC PL FLUOTAR | 2.5x/0.07 4x/0.13 |
|
| HC PL FLUOTAR | ||
| Optics (Lightsheet acquisition) | ||
| HC FLUOTAR | 5x/0.15 IMM DLS | |
| HC APO | L10x/0.30 W DLS | |
| HC APO | L20x/0.50 W DLS | |
| HC FLUOTAR | L25x/0.95 W DLS | |
| Experimental methods | ||
| Confocal | Z-stack | |
| Time Lapse | ||
| Multi-position | ||
| Tile scanning | ||
| Beaching-FRET | ||
| FLIM (Tau Sense) | ||
| Super Resolution (Lightning) | ||
| Lightsheet | Z-stack | |
| Time Lapse | ||
| Clearing | ||
| Software | ||
| LASX | ||
| Aivia Go3 | ||
Acknowledging the Technology Hub Facilities in Publications
Acknowledging the Technology Hub Facilities in Publications
In recognition of the essential support provided by the Technology Hub Facilities and in alignment with the values of The Technician Commitment and UKRI guidance on the recognition of specialist staff, we kindly request that all users acknowledge the contribution of the Technology Hub in any resulting publications, presentations, or grant applications. This helps ensure continued support and visibility for the facilities and the staff who make this work possible. Please include one of the following acknowledgment statements in your manuscripts or outputs:
-
General Acknowledgment (for use of equipment and/or technical support):
“We acknowledge the support of the [Facility] Technology Hub Facilities at the College of Medicine and Health, University of Birmingham, for providing access to equipment and technical expertise.”
-
Specialist Staff Contribution (if staff provided significant input):
“We thank [Staff Member’s Name] from the [Facility], Technology Hub Facilities, College of Medicine and Health, University of Birmingham, for their valuable technical assistance and input during this work.”
-
Co-authorship:
If a member of the Technology Hub staff has made a substantial intellectual or technical contribution to the research, we strongly encourage including them as a co-author, in line with standard authorship guidelines.
We appreciate your support in helping to raise the visibility and recognition of the essential contributions made by technical staff and facilities. If you are unsure how best to acknowledge the support received, please contact the Technology Hub team for guidance.
Contact us
Contact us
microscopyfacility@contacts.bham.ac.uk
techubfinance@contacts.bham.ac.uk
Research Facilities Manager
Dr Adriana Flores-Langarica
Email: A.FloresLangarica@bham.ac.uk
Microscopy Facility Academic Lead
Professor Steve Thomas
Email: s.thomas@bham.ac.uk
