Examination
You will be given a complete physical examination and your medical history will be taken. Scans using X-rays will be done to look at your disease status. Blood samples (approximately 40mls or 2 tablespoons per visit) will be taken and if you are a woman of childbearing potential, a pregnancy test will be done.
Consent
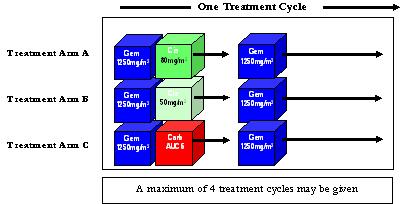
Once you have signed the consent document and the tests outlined above indicate it is safe for you to have the study chemotherapy, you will begin the treatment you have been allocated. Treatment consists of the chemotherapy drugs given through a drip into your vein on the 1st and 8th day of a 21-day treatment cycle. Up to 4 treatment cycles may be given.
Dosage calculation
The dose of gemcitabine and cisplatin are dependent on your height and weight that are used to calculate your body surface area (m2). The greater your body surface area the more milligrams of drug are required. However in the case of carboplatin, dose used is dependent on kidney function. This is because carboplatin is removed from your body principally by your kidneys; the better your kidneys work the higher the dose of carboplatin needed.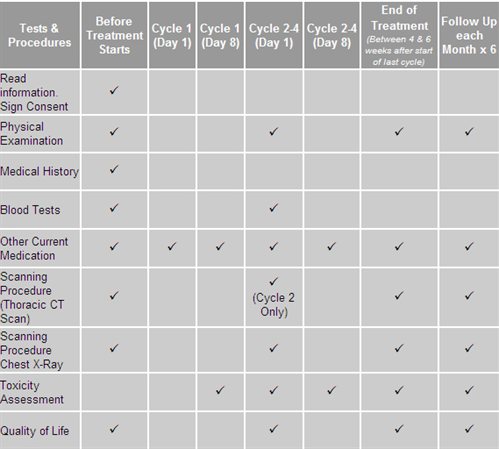
Day One
On Day One of each treatment cycle you will receive the chemotherapy drug Gemcitabine, (1250mg/m2) administered through a drip for approximately 30 mins.
You will then be given one of the following treatments you have been randomised to receive:- Cisplatin at 50 mg/m2 OR Cisplatin at 80 mg/m2 OR Carboplatin AUC 6 Administered through a drip for approximately one hour.
NOTE: If you receive cisplatin you will also receive extra fluids through the drip to help protect your kidneys from damage from the drug. Therefore the total time spent receiving cisplatin-based treatment is expected to be 6 hours and total time spent receiving carboplatin-based treatment is expected to be 1.5 hours.
Day Eight
On Day Eight of each treatment cycle you will receive the gemcitabine treatment only, again administered through a drip for approximately 30 mins.
Before each chemotherapy dose
Before each chemotherapy dose you will have a physical examination. You will be asked about any medical problems or changes in medication since the previous visit. Blood samples will be taken to see how the drugs are affecting you and to make sure it is still safe to continue to treat you. Also scans using X-rays will be taken to look at your disease status before treatment cycles 2, 3 and 4 begin.
End of treatment
At the end of the full treatment you will also have a physical exam, blood tests, and you will be asked about any medication changes or medical problems that you may have had during the previous month and a scan using X-rays will be taken, to look at your disease status. Any side effects continuing at that time will be followed until they have resolved or stabilized.
You will then be asked to return to clinic every 4 weeks up to a period of 6 months to find out if you have had any medical problems during the previous month. At these visits the doctor will perform a physical examination and a chest X-ray will be taken.