Scientists from the Universities of Birmingham and Oxford are collaborating on a pioneering tissue engineering project to 'grow' human bone. Ros Dodd finds out more from biomaterials expert Professor Liam Grover.
Losing a limb in a roadside bomb is terrible enough; developing the painful and further disabling disease heterotopic ossification (HO) – in which bone grows in abnormal places such as soft tissue – is an added trauma.
Unfortunately, this is what is happening to many servicemen who have lost arms and legs following IED (improvised explosive devices) explosions in Afghanistan and, previously, Iraq.
Such is the unpleasantness of HO that the ectopic bone can push its way through the tissue, causing severe discomfort. The massing of the bone around the amputated area can also prevent patients from being fitted with prosthetic limbs.

‘There are lots of servicemen who have come back from Iraq and Afghanistan who for some reason are developing large amounts of bone in their soft tissues; no one really knows why it happens, but it’s thought to relate to amputation and explosions such as those caused by IEDs,’ explains Professor Liam Grover, a worldleading figure in the development of biomaterials to repair and regenerate diseased and worn-out parts of the body, from ligaments to skin.
Liam and his research team – together with scientists from Oxford University – are working to develop a model of ossification, which will allow them to find a way to treat it effectively. At present, the ectopic bone is left to mature and then cut away; Liam wants to see if it can be dissolved or its formation prevented.
This approach also means not having to experiment on animals.
‘What we’re trying to do at the moment is to develop a model of the disease that doesn’t require the use of animals. This is because the existing models of ossification are sufficiently severe that it is not ethical to run large screening experiments on all of the agents that we think could be useful to disperse the newly forming bone,’ says Liam, whose work on this is being funded by the NC3Rs (the National Centre for the Replacement, Refinement and Reduction of Animals in Research).

‘So we are developing a model of bone formation – growing bone in culture dishes in my laboratory in very high amounts. This enables us to test lots of different components to see if we can get rid of the mineral. We want to see if we can dissolve it. ‘We’re also trying to learn about why the disease happens, because no one really knows for sure. If we can find this out, we can use that knowledge not only to relieve this particular issue, HO, but also to try to stimulate bone formation where there isn’t enough bone.’
Liam, Professor of Biomaterials Science in the School of Chemical Engineering at Birmingham, is an expert in the control of bone mineralisation, having developed a particular interest in how condensed phosphate species could play both an inhibitory and stimulatory role in bone mineralisation.
In 2006, he set up a research group at Birmingham aimed at finding novel ways to regenerate human tissue. The group is now engaged in a number of groundbreaking projects that are very close to transferring from the lab to the clinic.
His research interests encompass three main areas: developing materials that can replace bone and then degrade in response to the body regenerating its own bone formation; producing tissueengineered ligaments and bone, and using soft solids to create dressings that will prevent scarring in a range of tissues – for which he was last year awarded a £1.8 million Wellcome Trust grant.
Of course, using materials to repair damaged or diseased tissue is not new, but until fairly recently those materials were based on mechanical properties, with little attention being given to the composition. With the population living longer, there is greater-than-ever pressure to help people to stay healthy in old age – which means finding new, better ways to regenerate decrepit and diseased parts of the body.
Working with Birmingham Medical School, Liam and his team at TRAILab (Tissue Regeneration and Interface Lab) have developed cell therapy treatments for regenerating diseased or damaged bones, cartilage, ligaments, skin and even eyes, and to improve the body’s ability to heal itself.
‘As our knowledge of the interactions between materials and biology improves, we are able to design materials that can stimulate or prevent specific biological responses,’ says Liam.
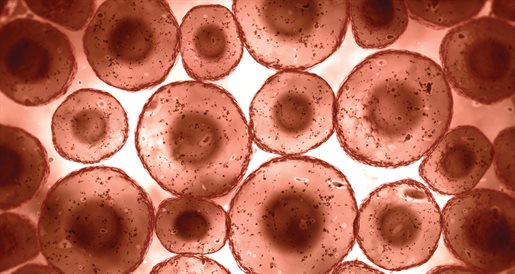
The materials he uses include patients’ blood and a population of their cells, which are then grown in the lab. They can then be encapsulated into a gel – which acts as a filler – and injected into the body. The cells will then excrete growth factors to encourage the body to heal itself.
Liam’s ligament and tendon research is now being trialled at Birmingham Orthopaedic Hospital. ‘We’re not clinically treating people; at this stage we’re just demonstrating that we can grow a person a new ligament in a clinically-relevant timeframe.’
The composition of body fluid – the minerals and proteins – gives ‘enormous scope’ to design materials that can be used to repair and regenerate, says Liam. However, relatively few can be used due to strict regulations. Nevertheless, scientists have moved into a new phase of discovery and treatment.
‘We have evolved from when we just implanted metallic materials into people,’ he comments. ‘Now it’s about linking the two – the body and the material.’
Although he admits he is first and foremost a pure scientist, Liam is also passionate about helping to prevent and treat illness and disability.
‘The application of my research has become very important to me,’ he says. ‘If we are successful in developing a new, effective treatment for HO, and finding out why it happens, I will be very happy. It will mean being able to alleviate some of the suffering experienced by patients who have this debilitating condition.’