The role of DNA damage response genes in haemopoietic malignancies
Overview
A tight regulation of cellular responses to DNA damage prevents generation of genomic alterations that can lead to tumour development. The integrity of these responses is particularly important in lymphoid progenitor cells that undergo developmentally regulated recombination of immune system genes. Cells with an intact DNA damage response (DDR) are capable of repairing a moderate level of DNA breaks and respond to an excess of unrepaired breaks by activation of apoptosis. The latter mechanism is utilized by the majority of DNA damaging chemotherapeutic agents. Therefore, defects in DNA damage response can lead not only to tumour development but also to tumour chemoresistance.
Our current research is focused on an elucidation of the role of Ataxia Telangiectasia Mutated (ATM) and other DNA damage response genes in the multistep process of leukaemia/lymphomagenesis. We are currently addressing a network of ATM dependent cellular responses and DNA repair defects such as RNaseH2 functional loss that can be targeted for treatment in chronic leukaemia (CLL) and other malignancies. This is facilitated by transgenic and primary tumour xenograft models. Our long-term goal is to translate the understanding of DNA damage response pathways into novel therapeutic strategies.
Our research group
The therapeutic exploitation of molecular defects within the DNA damage response has become an important paradigm in cancer treatment. ‘Synthetic lethality’ relies on the pharmacological inhibition of pathways upon which DDR-deficient cancer cells have become dependent for their survival. This induces an intolerable level of unrepaired DNA damage resulting in cell death, whilst sparing DDR-proficient normal cells. In previous work supported by CRUK and Bloodwise, we demonstrated that ATM-deficient CLL cells are highly sensitive to PARP inhibitors (PARPi) through the accumulation of DNA breaks that are normally repaired by an ATM-dependent DNA repair pathway.
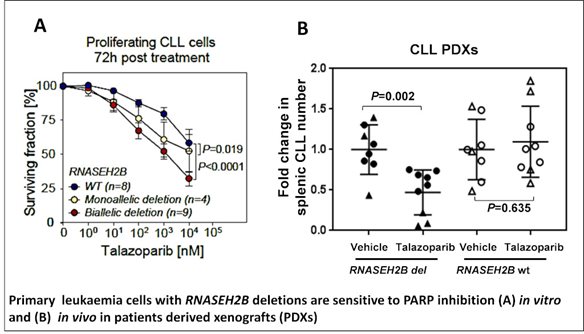
Similarly, we have demonstrated the utility of a synthetic lethal interaction between ATM/p53-deficiency and Ataxia Telangiectasia Related (ATR) inhibition to induce specific killing of treatment-resistant ATM/p53-defective CLL cells, both in vitro and in vivo. Furthermore, we have defined a role for the deubiquitylase, USP7, in regulating homologous recombination repair (HRR) and demonstrated that its inhibition could be used in a similar manner to ATR inhibitors to eliminate CLL cells independently of ATM or p53 status. Finally, we recently demonstrated that both heterozygous and homozygous deletion of the RNASEH2B gene, a subunit of RNaseH2 complex that resolves genome embedded nucleotides sensitises cancer cells to PARP inhibitors.
Our translational focus has supports a productive collaboration with the pharmaceutical industry. This led to the development of a clinical trial (PICLLE) utilising the PARPi Olaparib to treat refractory CLL, T-prolymphocytic leukaemia, and mantle cell lymphoma. In addition, through an ongoing interaction with AstraZeneca, we have initiated pre-clinical studies in patients with relapsed CLL and ATM/p53 dysfunction prior to initiation of a clinical trial that evaluates the combined use of ATR and BTK inhibitors.
- Identification of a network of ATM dependent cellular responses and DNA repair defects that can be targeted for treatment in chronic leukaemia (CLL) and other haemopoietic malignancies.
- Addressing different mechanisms of tumorigenesis and novel therapeutic approaches in mouse models of ATM deficient haematopoietic malignancies and xenograft models of leukaemias and lymphomas with DDR defects.
- Marston E, Weston V, Jesson J, Maina E, McConville C, Agathanggelou A, Skowronska A, Mapp K, Sameith K, Powell JE, Lawson S, Kearns P, Falciani F, Taylor M, Stankovic T. Stratification of paediatric ALL by in vitro cellular responses to DNA double strand breaks provides insight into the molecular mechanisms underlying clinical response Blood. 2009; 113(1):117-26.
- Victoria J Weston, Ceri E Oldreive, Anna Skowronska, David G Oscier, Guy Pratt, Martin JS Dyer, Graeme Smith, Judy E Powell, A Malcolm R Taylor, Paul AH Moss, Tatjana Stankovic. The PARP inhibitor olaparib suppresses growth of ATM mutant lymphoid tumour cells in vitro and in vivo. Blood. 2010;116(22):4578-87.
- Skowronska A, Austen B, Powell JE, Weston V, Oscier DG, Dyer MJS, Matutes E, Pratt G, Fegan C, Moss P, Taylor AM, Stankovic T. ATM germline heterozygosity does not play a role in CLL initiation but influences rapid disease progression through loss of the remaining ATM allele . Haematologica. 2012;97(1):142-6.
- Anna Skowronska, Anton Parker, Gulshanara Ahmed, Ceri Oldreive, Zadie Davis, Sue Richards, Martin Dyer, Estella Matutes E, David Gonzalez, AMR Taylor, Paul Moss, David Oscier, Tatjana Stankovic. Biallelic ATM Inactivation Significantly Reduces Survival in Patients Treated on UK CLL4 Trial. JCO 2012;30(36):4524-32.
- Da Costa D, Agathanggelou A, Perry T, Weston V, Petermann E, Zlatanou A, Oldreive C, Wei W, Stewart G, Longman J, Smith E, Kearns P, Knapp S, Stankovic T. BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia. Blood Cancer J. 2013 Jul 19;3:e126.
- Agathanggelou A, Weston VJ, Perry T, Davies NJ, Skowronska A, Payne DT, Fossey JS, Oldreive CE, Wei W, Pratt G, Parry H, Oscier D, Coles SJ, Hole PS, Darley RL, McMahon M, Hayes JD, Moss P, Stewart GS, Taylor AM, Stankovic T. Targeting the ATM-null phenotype in chronic lymphocytic leukemia with pro-oxidants 1. Haematologica 2015;100(8):1076-85.
- Oldreive CE, Skowronska A, Davies NJ, Parry H, Agathanggelou A, Krysov S, Packham G, Rudzki Z, Cronin L, Vrzalikova K, Murray P, Odintsova E, Pratt G, Taylor AM, Moss P, Stankovic T. T-cell numbers and subtypes control the duration and utility of primary chronic lymphocytic leukaemia xenograft in alymphoid mice, Disease Models, Mechanisms 2015;8(11):1401-12.
- Kwok M, Davies N, Agathanggelou A, Smith E, Oldreive C, Petermann E, Stewart G, Brown J, Lau A, Pratt G, Parry H, Taylor M, Moss P, Hillmen P, Stankovic T. ATR inhibition induces synthetic lethality and overcomes chemoresistance in TP53- or ATM-defective chronic lymphocytic leukemia cells. Blood 2016;127(5):582-95.
- Davies NJ, Kwok M, Gould C, Oldreive CE, Mao J, Parry H, Smith E, Agathanggelou A, Pratt G, Taylor AMR, Moss P, Griffiths M, Stankovic T. Dynamic changes in clonal cytogenetic architecture during progression of chronic lymphocytic leukemia in patients and patient-derived murine xenografts. Oncotarget 2017;8(27):44749-44760.
- Pratt G, Yap C, Oldreive C, Slade D, Bishop R, Griffiths M, Dyer MJS, Fegan C, Oscier D, Pettitt A, Matutes E, Devereux S, Allsup D, Bloor A, Hillmen P, Follows G, Rule S, Moss P, Stankovic T.A phase I trial of the PARP inhibitor olaparib in patients with relapsed chronic lymphocytic leukaemia, T-prolymphocytic leukaemia or mantle cell lymphoma. BJH 182(3):429-433.
- Agathanggelou A, Smith E, Davies NJ, Kwok M, Zlatanou A, Oldreive CE, Mao J, Da Costa D, Yadollahi S, Perry T, Kearns P, Skowronska A, Yates E, Parry H, Hillmen P, Reverdy C, Delansorne R, Paneesha S, Pratt G, Moss P, Taylor AMR, Stewart GS, Stankovic T. USP7 inhibition alters homologous recombination repair and targets CLL cells independent of ATM/p53 functional status. Blood 2017;130(2):156-166.
- Zimmermann M, Murina O, Reijns MAM, Agathanggelou A, Challis R, Tarnauskaitė Ž, Muir M, Fluteau A, Aregger M, McEwan A, Yuan W, Clarke M, Lambros MB, Paneesha S, Moss P, Chandrashekhar M, Angers S, Moffat J, Brunton VG, Hart T, de Bono J, Stankovic T, Jackson AP, Durocher D.CRISPR screens identify genomic ribonucleotides as a source of PARP-trapping lesions. Nature. 2018 Jul;559(7713):285-289.
- Oldreive CE, Byrd PJ, Stewart GS, Taylor AJ, Farhat S, Skowronska A, Smith E, Raghavan M, Janic D, Dokmanovic L, Clokie S, Davies N, Kwok M, Pratt G, Paneesha S, Moss P, Stankovic T, Taylor M.PALB2 variant status in hematopoietic malignancies – a potential therapeutic target? L&L 2019, 7:1-4.