We now have the opportunity to follow-up the same 1,290 patients that took part in the original BALLETS study. This additional research will give us an even better understanding of longer-term health outcomes for people who have a mildly abnormal Liver Function Test (LFT). The original BALLETS study followed-up patients two years after they had a mildly abnormal LFT to see if they developed liver-related health problems. We will be able to see if the same people developed liver-related problems after a period of 15 years.
Methods
In the BALLETS follow-up study, we will be electronically linking patients' NHS numbers with two national databases to find out if they developed any liver-related health problems during the period 2007-2020. The two datasets we will be using are:
- Hospital Episode Statistics (HES) – This database contains details of all emergency and elective inpatient admissions, outpatient appointments, and A&E attendances funded by the NHS in England. This dataset will allow us to identify patients with inpatient admissions or outpatient attendances for liver disease.
- Office of National Statistics (ONS) – The ONS mortality database captures the date, location and cause of death for all individuals in the UK. This dataset will allow us to identify all patients who have died since taking part in the original BALLETS study.
All patients who were part of the original BALLETS study have the option of opting-out of the study.
Linking the Data
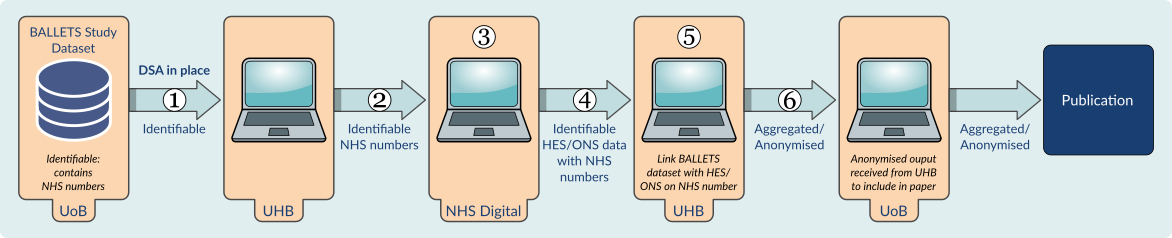
There are several stages to linking the data and the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust have strict governance policies to make sure that your data is safe.
- Data from patients who have requested to opt-out of the BALLETS study will be removed from the BALLETS study dataset.
- The University of Birmingham send NHS numbers and Dates of Birth from the patients in the BALLETS study to University Hospitals Birmingham NHS Foundation Trust.
- The team at University Hospitals Birmingham make a request to NHS Digital for data for the patients in the original cohort. For each patient, the BALLETS study team would only request data on specific items. Each field of data requested from NHS Digital has to be justified (i.e. only data relating to liver disease or liver related-conditions).
- Once the request for data has been approved, patients’ NHS numbers are sent to NHS Digital – patient names are not sent. This process is very secure.
- NHS Digital send the data requested to the lead researcher, Dr Kitty Reeves, who is based at University Hospitals Birmingham NHS Foundation Trust. The data are sent in encrypted files, and there are strict governance policies regarding data handling and storage. Kitty Reeves receives annual training on data handling and data security.
- Kitty Reeves is the only member of the research team to see individual patient NHS numbers and the data from Hospital Episode Statistics (HES) and Office of National Statistics (ONS) linked to those patients.
- Other members of the research team will only be sent aggregated data (e.g. 5% of patients from the original cohort developed xx).
Consent
All people who took part in the original BALLETS study agreed to be followed up in the future. The consent form included the statement: “We will also ask your permission to keep in touch with you after the study has ended, as we are hoping that we will be able to follow-up the participants of this study for a number of years, e.g. to find out if any participants have developed liver disease since the study has ended.”
However, at the time we did not make it explicit that we would be linking data in this way.
Why Can't We Contact People Now?
It would not be feasible for us to contact all 1,290 patients that signed-up to the original BALLETS study for two main reasons:
- Some people will have died and attempts to contact the patients may be distressing for family members.
- Some people may have moved house.
Because it is not feasible for us to contact each patient that agreed to be part of the study, we applied to the Confidentiality Advisory Group, which is organised by the Health Research Authority.
The Confidentiality Advisory Group (CAG) Panel reviewed our study and provided a legal basis for us (the data controllers) to share confidential patient information without consent.
What We Will Do With the Data
This is an important study that will help us understand how we can provide the best quality care for people who have no known liver condition and who have mildly abnormal Liver Function Tests.
We will publish the findings of this study in academic journals. We will also share the findings in our networks with academic, healthcare and health service colleagues through using a range of approaches, such as using social media platforms (Twitter) and through the ARC WM News blog.