Research in the section is focussed across the spectrum of materials chemistry from inorganic materials to organic, inorganic/organic hybrid and polymeric materials. We have interests that span the areas of material design, synthesis and characterisation that are focussed on applications as diverse as battery and hydrogen storage/fuel cell materials, sustainably plastics, porous solids for catalysis, gas storage, nuclear waste remediation, nanoscience, drug delivery and healthcare technologies. Alongside these activities, we have a significant focus on recycling and sustainability – in particular in the areas of batteries and plastics
We make extensive use of synchrotron and neutron diffraction techniques and have strong links to these central facilities as well as collaborations across the University in areas that include Materials and Metallurgy, Environmental Science, Chemical Engineering and Healthcare Technologies.
Research section leader
Areas of interest
Polymerisation and depolymerisation catalysis, sustainable polymer chemistry, degradable polymers, 3D printing, biomedical materials, organic catalysis, stereochemistry in polymeric materials.
Representative publication: Independent Control of Elastomer Properties through Stereocontrolled Synthesis
Angew. Chem. Int. Ed. (2016) 55, 13076 – 13080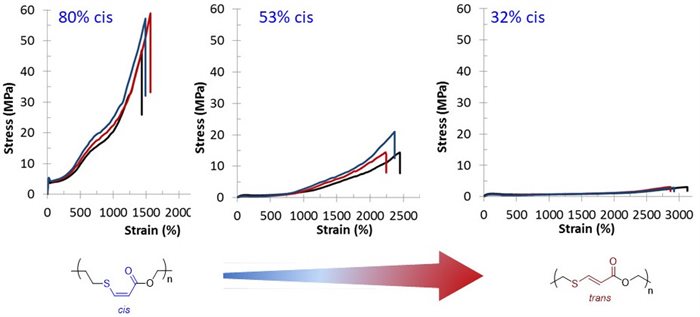 Elastomers are typically tri- or multi-block copolymers that owe their properties to phase separation of the polymer blocks to form hard and soft domains that infer the strength and flexibility respectively. Conversely, natural rubber and gutta percha display different mechanical properties as a result of the different stereochemistry of the double bonds in the polymer backbone. Using stereospecific, organocatalyzed click chemistry the materials properties can be controlled which allows the decoupling of mechanical properties, physical properties and materials’ degradation to provides a new design space for elastomers.
Elastomers are typically tri- or multi-block copolymers that owe their properties to phase separation of the polymer blocks to form hard and soft domains that infer the strength and flexibility respectively. Conversely, natural rubber and gutta percha display different mechanical properties as a result of the different stereochemistry of the double bonds in the polymer backbone. Using stereospecific, organocatalyzed click chemistry the materials properties can be controlled which allows the decoupling of mechanical properties, physical properties and materials’ degradation to provides a new design space for elastomers.
Research unit members
Areas of interest
lithium- and sodium-ion batteries, ion-exchangers, electrochemistry, recycling, solid-state materials synthesis, synchrotron-based characterization, in situ studies of functional behaviour.
Representative paper: Tracking Sodium-Antimonide Phase Transformations in Sodium-Ion Anodes: Insights from Operando Pair Distribution Function Analysis and Solid-State NMR Spectroscopy
Phoebe K. Allan, John M. Griffin, Ali Darwiche, Olaf J. Borkiewicz, Kamila M. Wiaderek, Karena W. Chapman, Andrew J. Morris, Peter J. Chupas, Laure Monconduit, and Clare P. Grey.
Journal of the American Chemical Society,
2016, 138 , 7 2352, DOI: 10.1021/jacs.5b13273
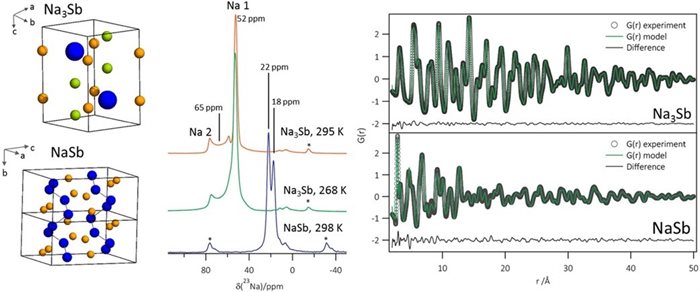 Antimony is a promising high-capacity anode material for sodium-ion batteries. However, many of the (dis)charge products are amorphous, meaning that the way in which sodium is stored in the material is not well understood. This paper uses solid-state NMR and (X-ray) pair distribution function analysis - methods which are sensitive to the local structure - to shed light upon the amorphous discharge products and hence the reasons for the excellent electrochemical performance of antimony anodes.
Antimony is a promising high-capacity anode material for sodium-ion batteries. However, many of the (dis)charge products are amorphous, meaning that the way in which sodium is stored in the material is not well understood. This paper uses solid-state NMR and (X-ray) pair distribution function analysis - methods which are sensitive to the local structure - to shed light upon the amorphous discharge products and hence the reasons for the excellent electrochemical performance of antimony anodes.

Professor of Strategic Elements and Materials Sustainability
Co-Director of the Birmingham Centre for Strategic Elements and Critical Materials
School of Chemistry
- Telephone
- +44 (0) 121 414 4447
- Email
- p.a.anderson@bham.ac.uk
Areas of interest
Critical materials, endangered elements, hydrogen storage materials, metal-organic frameworks, microporous materials, materials for energy storage and conversion, non-oxide solid electrolytes, zeolites
Representative paper: Synthesis and Characterization of Two New Amide Chloride Compounds: Potential H2 Storage Materials
R. A. Davies, D. R. Hewett and P. A. Anderson, Int. J. Hydrogen Energy, 2015, 40, 3001–3005. DOI: 10.1016/j.ijhydene.2014.12.044 Despite the promising volumetric and gravimetric hydrogen storage capacities of many complex hydrides, reaction kinetics remain too slow for on-board hydrogen storage in automotive applications. Previously we showed that a range of amide halide compounds exhibited both improved kinetics of hydrogen release/reabsorption and elimination of ammonia release, but at the cost of a reduction in gravimetric capacity through the incorporation of halide. This paper reports the identification of two new amide chloride phases in which the chloride content has effectively been halved. For both new phases, this gravimetric gain was achieved with improvements in both desorption and rehydrogenation properties.
Despite the promising volumetric and gravimetric hydrogen storage capacities of many complex hydrides, reaction kinetics remain too slow for on-board hydrogen storage in automotive applications. Previously we showed that a range of amide halide compounds exhibited both improved kinetics of hydrogen release/reabsorption and elimination of ammonia release, but at the cost of a reduction in gravimetric capacity through the incorporation of halide. This paper reports the identification of two new amide chloride phases in which the chloride content has effectively been halved. For both new phases, this gravimetric gain was achieved with improvements in both desorption and rehydrogenation properties.
Areas of interest
Polymer chemistry, crystallisation-driven self-assembly, nanomaterials, cell engineering, cell-based materials, bio-orthogonal chemistry
Representative paper: Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties
M.C. Arno*, M. Inam, A.C. Weems, Z. Li, A.L.A. Binch, C.I. Platt, S.M. Richardson, J.A. Hoyland, A.P. Dove*, R.K. O’Reilly*. Nat. Commun, 2020, 11, 1420
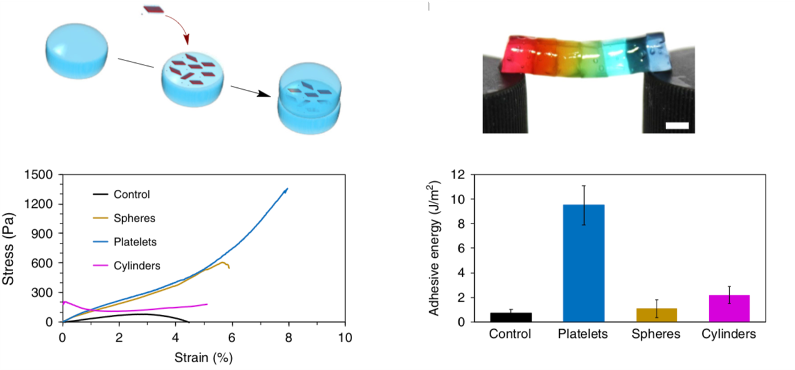
The ability to control nanostructure shape and dimensions presents opportunities to design materials in which their macroscopic properties are dependent upon the nature of the nanoparticle. However, the exploitation of the potential shape-dependent performance of nanomaterials has, to date, been limited. This paper demonstrates that nanoparticle shape is a critical consideration in the determination of nanocomposite hydrogel properties, including adhesion and mechanical strength. This study opens the doors to a change in direction in the field of gel nanocomposites, where nanoparticle shape plays an important role in tuning mechanical properties.
Areas of interest
Quantum materials, magnetic materials, materials discovery, structure-property relationships, X-ray and neutron scattering, muon spectroscopy
Representative paper: From magnetic order to quantum disorder in the Zn-barlowite series of S = 1/2 kagomé antiferromagnets
Katherine Tustain, Brendan Ward-O’Brien, Fabrice Bert, Tianheng Han, Hubertus Luetkens, Tom Lancaster, Benjamin M. Huddart, Peter J. Baker and Lucy Clark. npj Quantum Materials, 2020, 5, 74 (https://www.nature.com/articles/s41535-020-00276-4)
Quantum spin liquids (QSLs) occur when the magnetic moments in a material act like a liquid, remaining dynamic and disordered even at absolute zero. Their highly entangled nature should give rise to exotic physical phenomena that could have applications in quantum computing, and developments in QSLs may also help in the understanding of high-temperature superconductivity. The theoretical consensus is that the arrangement of magnetic moments in a two-dimensional corner-sharing triangular network, known as a kagomé network, is a likely candidate for a QSL. However, the true nature of such a state remains elusive. In this paper, we used a combination of materials synthesis, neutron diffraction, muon spectroscopy and density functional theory to map the magnetic phase diagram of a material known as Zn-barlowite, revealing the onset of a possible QSL phase.
Areas of interest
Energy storage, hydrogen storage, solid state chemistry, metal-nitrogen-hydrogen materials, ammonia synthesis and decomposition, ionic conduction, X-ray and neutron powder diffraction, in situ characterisation.
Representative paper: Ammonia decomposition catalysis using non-stoichiometric lithium imide
JW Makepeace, TJ Wood, HMA Hunter, MO Jones, WIF David, Chemical Science, 2015, 6, 3805-3815
(https://pubs.rsc.org/en/content/articlehtml/2015/sc/c5sc00205b)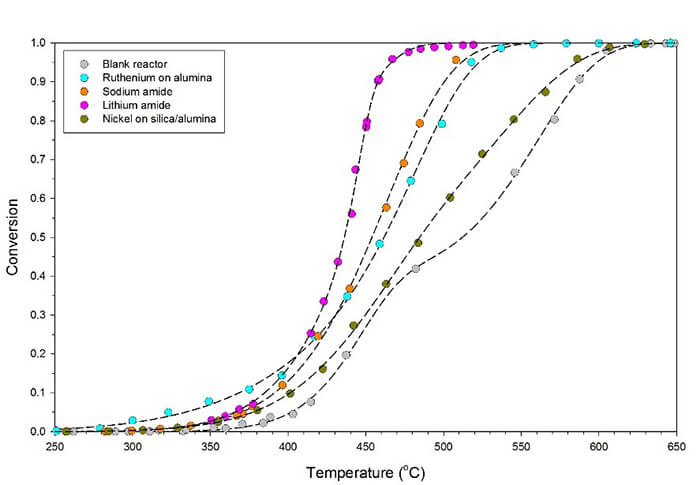 Ammonia is an attractive answer to the question of how best to store and transport hydrogen so it can be used as a sustainable fuel. However, the catalytic decomposition of ammonia to release its stored hydrogen using transition metals (ruthenium is the most active metal) typically requires very high temperatures. This paper details a new approach to ammonia decomposition catalysis using lithium imide (Li2NH), showing a decrease in the temperature of 90% conversion of around 50°C compared to ruthenium. In this work, in situ neutron powder diffraction was used to demonstrate that under operating conditions the catalyst adopts a non-stoichiometric mixed amide-imide phase. Furthermore, H-D isotope exchange reactions showed that the entire bulk of the catalyst interacts with the ammonia as it decomposes, in contrast to common surface-only catalytic cycles.
Ammonia is an attractive answer to the question of how best to store and transport hydrogen so it can be used as a sustainable fuel. However, the catalytic decomposition of ammonia to release its stored hydrogen using transition metals (ruthenium is the most active metal) typically requires very high temperatures. This paper details a new approach to ammonia decomposition catalysis using lithium imide (Li2NH), showing a decrease in the temperature of 90% conversion of around 50°C compared to ruthenium. In this work, in situ neutron powder diffraction was used to demonstrate that under operating conditions the catalyst adopts a non-stoichiometric mixed amide-imide phase. Furthermore, H-D isotope exchange reactions showed that the entire bulk of the catalyst interacts with the ammonia as it decomposes, in contrast to common surface-only catalytic cycles.
Areas of interest
Polymer synthesis, self-assembly, nanoparticles, catalysis, soft matter and DNA.
Representative paper: Confinement of Therapeutic Enzymes in Selectively Permeable Polymer Vesicles by Polymerization-Induced Self-Assembly (PISA) Reduces Antibody Binding and Proteolytic Susceptibility
Lewis D. Blackman, Spyridon Varlas, Maria C. Arno, Zachary H. Houston, Nicholas L. Fletcher, Kristofer J. Thurecht, Muhammad Hasan, Matthew I. Gibson, and Rachel K. O’Reilly. ACS Central Science, 2018, 4, 718
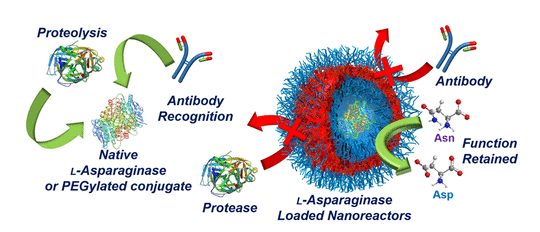 This paper highlights an alternative to PEGylaton through protection of a therapeutic enzyme using a polymer capsule, prepared in a scalable and precise way. This represents a new direction for the growing field of polymerisation induced self-assembly.
This paper highlights an alternative to PEGylaton through protection of a therapeutic enzyme using a polymer capsule, prepared in a scalable and precise way. This represents a new direction for the growing field of polymerisation induced self-assembly.
Areas of interest
Nanoscale Science; Noncovalent Bonding; Self-Assembly; Self-Organisation; C60; Electron Beam Resists; Liquid Crystals; Self-Assembled Monolayers; Nano-Tribology
Representative paper: Novel polystyrene sulfonate-silica microspheres as a carrier of a water soluble inorganic salt (KCl) for its sustained release, via a dual-release mechanism
C. Sui, J.A. Preece, Z. Zhang, RSC Advances, 2017, 7, 478-481.
 This paper reports for the first time the encapsulation of a water soluble inorganic salt in a hybrid organic-inorganic microcapsule. By careful tuning of the microcapsule chemistry it proved possible to modulate the release of the salt, which we propose involves two stages: initially by leaching out as complex with an encapsulated polyanion, and subsequently as the free salt. The work has been patented, and industry has shown an interest.
This paper reports for the first time the encapsulation of a water soluble inorganic salt in a hybrid organic-inorganic microcapsule. By careful tuning of the microcapsule chemistry it proved possible to modulate the release of the salt, which we propose involves two stages: initially by leaching out as complex with an encapsulated polyanion, and subsequently as the free salt. The work has been patented, and industry has shown an interest.
Areas of interest
Green Chemistry; Sustainability; Biomass; energy (fuel cells, batteries); water purification
Representative paper: Mechanistic insights into porous carbons from gelatin
A.E. Danks, M.J. Hollamby, B. Hammouda, D.C. Fletcher; F. Johnston-Banks; S.R. Rogers, Z. Schnepp; J. Mater. Chem. A 5, 11644-11651, 2017.
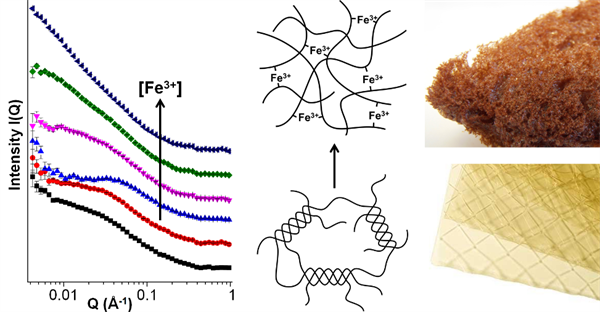 In this paper we show how the biopolymer gelatin can be utilised to produce functional carbon foams with multimodal porosity. The mechanism of the formation of these foams is assessed through the use of small angle neutron scattering alongside other techniques. We illustrate that the choice of metal nitrate can be exploited to control foam macrostructure, attributed to synergistic interaction of metal ions with the gelatin polypeptide, which changes the viscoelastic properties.
In this paper we show how the biopolymer gelatin can be utilised to produce functional carbon foams with multimodal porosity. The mechanism of the formation of these foams is assessed through the use of small angle neutron scattering alongside other techniques. We illustrate that the choice of metal nitrate can be exploited to control foam macrostructure, attributed to synergistic interaction of metal ions with the gelatin polypeptide, which changes the viscoelastic properties.
Representative paper: Combined Experimental and Computational Study of Ce-Doped La3Zr2Li7O12 Garnet Solid-State Electrolyte
Bo Dong, Stephen R. Yeandel, Pooja Goddard, and Peter R. Slater; Chemistry of Materials 32, 215-223, 2020
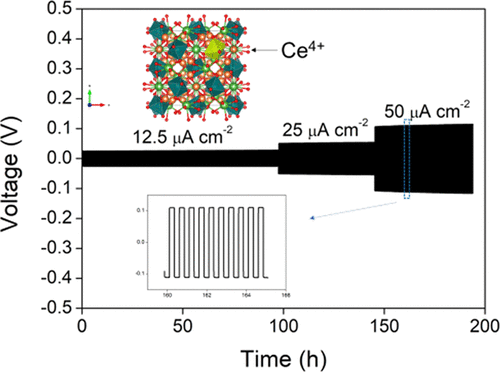
In this paper we show that Ce incorporation into La3Zr2Li7O12 leads to improved conductivity, as well as reducing the interfacial resistance in contact with Li metal. The latter can be attributed to the formation of a conducting interface, which offers significant potential benefits in terms of operation of garnet electrolytes in all solid state batteries.
There are no results that match your search
Areas of interest
Polymer Synthesis - Nanotechnology - Supramolecular Science - Smart Materials - Antimicrobials - Drug Delivery
Representative paper: In Situ Functionalized Polymers for siRNA Delivery
Angew. Chem., Int. Ed. (2016), 55, 7492–7495
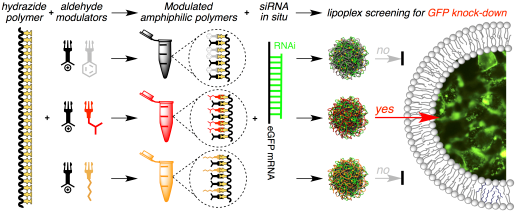 A new method is reported herein for screening the biological activity of functional polymers across a consistent degree of polymerization and in situ, that is, under aqueous conditions and without purification/isolation of candidate polymers. In brief, the chemical functionality of a poly(acryloyl hydrazide) scaffold was activated under aqueous conditions using readily available aldehydes to obtain amphiphilic polymers. The transport activity of the resulting polymers can be evaluated in situ using model membranes and living cells without the need for tedious isolation and purification steps. This technology allowed the rapid identification of a supramolecular polymeric vector with excellent efficiency and reproducibility for the delivery of siRNA into human cells (HeLa‐EGFP). The reported method constitutes a blueprint for the high‐throughput screening and future discovery of new polymeric functional materials with important biological applications.
A new method is reported herein for screening the biological activity of functional polymers across a consistent degree of polymerization and in situ, that is, under aqueous conditions and without purification/isolation of candidate polymers. In brief, the chemical functionality of a poly(acryloyl hydrazide) scaffold was activated under aqueous conditions using readily available aldehydes to obtain amphiphilic polymers. The transport activity of the resulting polymers can be evaluated in situ using model membranes and living cells without the need for tedious isolation and purification steps. This technology allowed the rapid identification of a supramolecular polymeric vector with excellent efficiency and reproducibility for the delivery of siRNA into human cells (HeLa‐EGFP). The reported method constitutes a blueprint for the high‐throughput screening and future discovery of new polymeric functional materials with important biological applications.
Areas of interest
Functional materials, porous materials, metal-organic frameworks, molecular conductors, crystallography, energetics, in situ X-ray diffraction, crystallisation, formation mechanisms, high pressure, synchrotron techniques.
Representative paper: In-Situ Observation of Successive Crystallisations and Metastable Intermediates in Metal-Organic Framework Formation
H. H.-M. Yeung,* Y. Wu, S. Henke, A. K. Cheetham, D. O’Hare, R. I. Walton
Angewandte Chemie, International Edition 2016 55, 2012-2016.
DOI: 10.1002/ange.201508763
Understanding the driving forces controlling crystallization is essential for the efficient synthesis and design of new materials, particularly metal–organic frameworks (MOFs), where mild solvothermal synthesis often allows access to various phases from the same reagents. Using high‐energy in situ synchrotron X‐ray powder diffraction, we monitor the crystallization of lithium tartrate MOFs, observing the successive crystallization and dissolution of three competing phases in one reaction. By determining rate constants and activation energies, we fully quantify the reaction energy landscape, gaining important predictive power for the choice of reaction conditions. Different reaction rates are explained by the structural relationships between the products and the reactants; larger changes in conformation result in higher activation energies.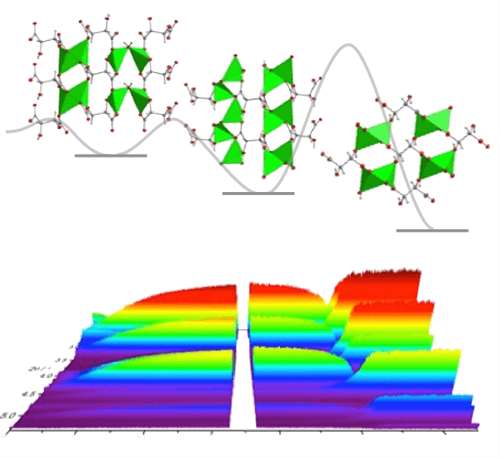
Contact
Enquiries about specific aspects of their research areas should be addressed to individual research group leaders. For more general enquiries about working with the Materials Chemistry Section, please contact the Head of the Section, Professor Andrew Dove. Information on various postgraduate (PhD and Masters) degree opportunities can be found on our postgraduate opportunities page.