New routes for targeted assembly
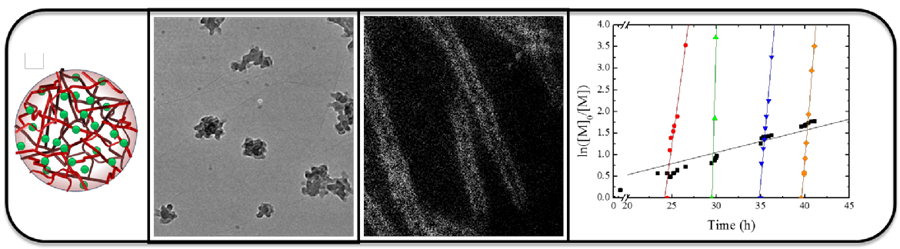
There is great interest in the development of new methodologies for the preparation of self-assembled nanostructures. Most commonly self-assembly is dominated by the hydrophobic effect and the hydrophobic:hydrophilic block ratio determines the overall morphology of the polymeric nanostructure. In recent years we have been interested in exploring how we can use other driving forces such as chain end control to drive the formation of well-defined nanostructures.
Furthermore, we are interested in blending of copolymer blocks can be used to access a range of micelle morphologies with minimal synthetic effort.
We have been able to show that a simple blending protocol for diblock copolymers with identical block lengths but varying hydrophobic monomer incorporation can be used to overcome thermodynamic and kinetic limitations, whilst being robust enough to suppress synthetic difficulties producing well defined tuneable nanostructures. We have demonstrated that a range of equilibrium structures can be produced from blending just two polymers; these blended structures are identical to those formed from the pure synthetic analogue at the same composition. We established for diblock copolymers this simple blending strategy offers great potential as simple, cheap yet robust route to well-defined functional spherical structures on the nanoscale.
Selected publications
Blending block copolymer micelles in solution; obstacles of blending, D. B. Wright, J. P. Patterson, N. C. Gianneschi, C. Chassenieux, O. Colombani and R.l K. O'Reilly, Polym. Chem., 2016, 7, 1577-1583, DOI: 10.1039/C5PY02006A
The Copolymer Blending Method: A New Approach for Targeted Assembly of Micellar Nanoparticles, D. B. Wright, J. P. Patterson, A. Pitto-Barry, A. Lu, N. Kirby, N. C. Gianneschi, C. Chassenieux, O. Colombani, and R. K. O’Reilly, Macromolecules, 2015, 48, 6516–6522. DOI: 10.1021/acs.macromol.5b01426
Self-assembly of Hydrophilic Homopolymers: A Matter of End Groups, J. Du, H. Willcock, J.P. Patterson, R. K. O'Reilly, Small, 2011, 14, 2070-2080. DOI: 10.1002/smll.201100382.