The significant breakthrough in making 2,5-Dimethylfuran (DMF) and 2-Methylfuran (MF) reported by Nature and Science respectively in 2007 has made it being considered as new biofuel candidates but previously there was no knowledge about its combustion and emissions in power systems. This world-first research of DMF at University of Birmingham following up the discovery has included a systematic experimental and modelling investigation of DMF as bio-fuel in the direct injection spark-ignition engine. This was followed by a series of detailed investigation of MF and other furanic fuels as alternative bio-fuel candidates.
These investigations have covered the spray, combustion, emissions, simulation, optical diagnostics and hydrocarbon gas speciation. The results have demonstrated the advantages of DMF’s higher energy density compared with ethanol, good anti-knock property, lower particulate matter emissions and potentially better cold-start performance. Similarly, the higher energy density of MF compared with ethanol provide advantages and exhibits good anti-knock property and higher indicated thermal efficiencies. The investigation has quantified its laminar flame speed under various conditions, unregulated emissions and toxicity concerning its impact on the environment. Spray and combustion models for DMF/MF application in SI engines have been developed and validated by quantified experimental results.
The investigators have collaborated with various industrial partners including Jaguar Cars Ltd, Innospec and Green Fuels. Academic collaboration has also been made with Xi’an Jiaotong University and Tsinghua University, both in China. This research at the University of Birmingham has also involved the School of Chemical Engineering and the School of Bio-science where the production of DMF using super critical water technology and the toxicity of DMF were investigated, respectively. The main results of the research have so far been published in over 20 peer-reviewed prestigious international journals, as briefly summarised below.
Spray
The spray characteristics of pure DMF, gasoline, ethanol and DMF-gasoline blends have been studied by the Schlieren, shadowgraphy and PDPA techniques in terms of spray penetration, droplet velocities and size distributions in order to investigate the spray behaviour of the new potential fuel, DMF. The spray characteristics of DMF are particularly favourable. The spray pattern when using DMF is very similar to that with gasoline. The DMF spray also has a negligibly larger droplet size than the gasoline spray (Tian et al. 2010b). Next, DMF and its blend with gasoline have a slightly lower mean velocity than gasoline. For most of the cases, the difference is less than 10m/s. The injection pressure plays an important role in the droplet size. For DMF, gasoline and ethanol, the SMDs decrease to 8.8, 8.3 and 7µm, respectively when the injection pressure increases from 50 to 150 bar. Increasing the injection pressure benefits the DMF spray more so than ethanol's. It is concluded that spray characteristics of DMF is more close to that of gasoline than that of ethanol.
The near-field and far-field spray characteristics of pure MF, isooctane and ethanol have been studied by high-speed using Shadowgraphy technique to investigate the behaviour of the potential fuel, MF (Wang et al.). MF injection exclusively exhibited a bubble-like spray tip at the start of injection for injection pressures upto 400 bar as compared to 300 bar for ethanol owing to the higher surface tension of MF. Isooctane did not exhibit any such characteristics. MF showed lowest penetration velocities amongst the three and wider macro spray angles compared to ethanol. Under flash boiling conditions, MF showed better primary spray breakup behaviour compared to ethanol and isooctane owing to its high vapour pressure. At flare boiling conditions, MF was completely atomized immediately after the exit from nozzle and had wider sprays compared to ethanol and isooctane.
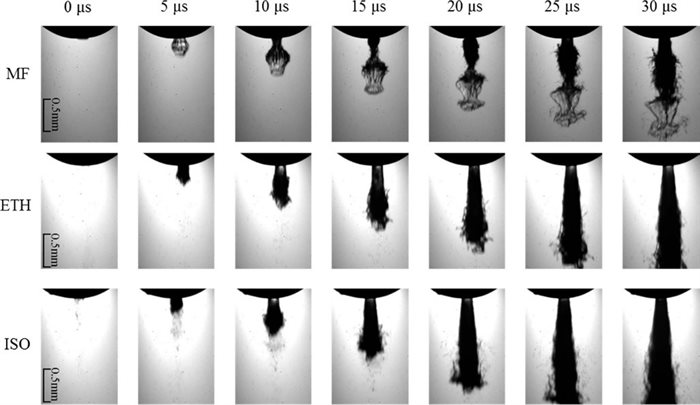
Figure 1. Backlight shadowgraph imaging of spray behaviour
Optical study of flame
The flame propagation characteristics of 2,5-dimethylfuran (DMF) were performed using the Schlieren optical method (Tian et al.). DMF’s laminar flame characteristics from a quiescent homogenous mixture in a constant volume vessel using the schlieren method. Of concern is the outwardly propagating spherical laminar flame speed, the Markstein length and the laminar burning velocity of DMF for a range of equivalence ratios (ϕ = 1.6) and initial temperatures (50-100ºC). DMF’s performance was benchmarked against gasoline and then compared to the current biofuel leader, ethanol. It is found that in terms of flame speed, DMF is similar to gasoline but slower than ethanol. Meanwhile, DMF shows the slowest laminar burning velocity (up to 10% slower than gasoline and 20-40% slower than ethanol at stoichiometry). These behaviors are subject to the effect of equivalence ratio and initial temperature. Flame propagation of DMF in the direct injection SI engine is studied in the optical engine. It is shown that DMF-gasoline dual-injection combustion has higher flame propagation speed and shorter combustion duration than baseline 100% gasoline PFI. The flame luminance of DMF-gasoline is much higher than ethanol-gasoline and pure gasoline. The engine load conditions have less influence on DMF-gasoline dual-injection flame propagation speed. The results of the flame propagation process and flame speed prove that DMF's existence in the fuel blends will bring the combustion closer to that with pure DMF, even when using low percentages.
The flame propagation characteristics of 2-methylfuran (MF) were investigated using the high-speed Schlieren optical method and compared (Ma et al.). Properties investigated include flame propagation velocity, Markstein length and unburnt laminar flame speed of MF for a range of equivalence ratio (0.7-1.4) and unburnt temperature (60 – 90⁰C). The performance of MF was benchmarked against isooctane and DMF. MF exhibited a flame speed upto 50% faster than isooctane and 30% faster than DMF. The flame propagation characteristics of MF-isooctane blends were also studied and showed that the blended fuels tended to be less stable than the corresponding isooctane flames but more stable than MF flames. Flame propagation of MF in a DISI engine was studied using the single cylinder optical engine (Ma et al.). MF is shown to combust significantly faster than DMF and isooctane with an earlier combustion phase and a shorter combustion duration compared to both fuels. The differences in the averaged flame propagation between DMF and isooctane was limited at lower temperatures and correlates well with the pressure trace and combustion phasing. OH-LIF signals correlated with MFB results well for the three fuels and showed that the OH measurements could be used as qualitative indicator of the MFB.
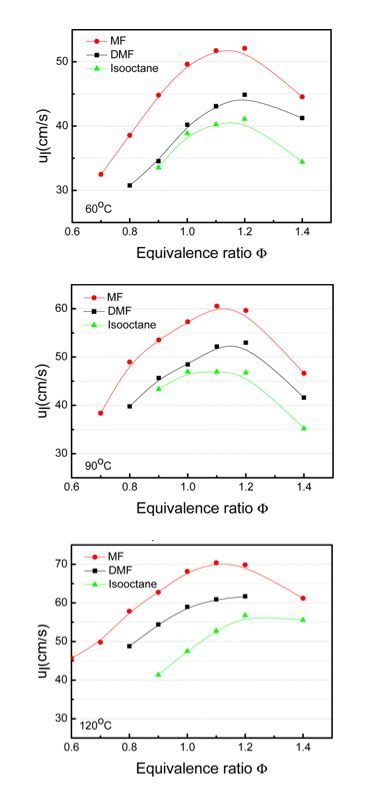
Figure 2. Laminar flame speed comparison between MF, DMF and isooctane
Additionally, the cellular instability and self-acceleration of premixed flames are inherent combustion characteristics commonly found in fuels. These key combustion characteristics serve as the foundation for refining combustion models and theoretical bases for engines design. In the case of small-scale flames, the cellular instability of premixed flames mainly arises from two types of instabilities: the thermo-diffusive and fluid dynamics instabilities. Flame cellular instability depends on the physicochemical properties of the fuel, and its study is predominantly based on experimental methods observing flame development. However, at present, there is scant research on the geometric features of cellular structures and a lack of clear quantitative description. Cellular instability can increase the flame speed and may cause laminar flames to become turbulent. Results show that the premixed flame propagation can be divided to three stages, namely, the steady combustion stage, the transition acceleration stage, and the saturated acceleration stage, respectively.
The study at the University of Birmingham employs a combination of experimental measurements, numerical calculations, and theoretical analysis, targeting three gases of different weights—hydrogen, methane, and propane—as fuel. It conducts quantitative studies on the instability and self-acceleration of premixed flames under different initial temperatures, pressures, and equivalence ratio conditions. According to the image processing concept proposed (Xu et al., 2017.02) at the University of Birmingham, the evolution process of flame cellular structures and the changing patterns of their characteristic parameters are analyzed by a developed software. First, the original flame image will go through sharpening, binaryzation, and noise reduction. Then, the information of each zone will be extracted where the blue star refers to the geometry centre of each zone. Based on the projection principle of sphere, the morphology of the cells in the 2D flame periphery can be regarded as the projection of actual cell on the spherical surface. Meanwhile, the geometry patterns in flame periphery will be fitted by oval, and the corresponding curvatures will also be calculated. The obtained size and curvature database will be used to generate the distribution function of cell size, and curvature can be derived for further analysis. It establishes a method to quantitatively determine the critical point of full flame cellularization (which is also the starting point of flame self-acceleration) and reveals the mechanisms of flame instability and self-acceleration. The study also acquires the corresponding flame acceleration indices and fractal dimensions. Finally, it establishes an empirical model of the combustion speed during self-acceleration through comprehensive analysis, contributing to the fundamental research of combustion science and the application of bio-substitute fuels in ICEs.
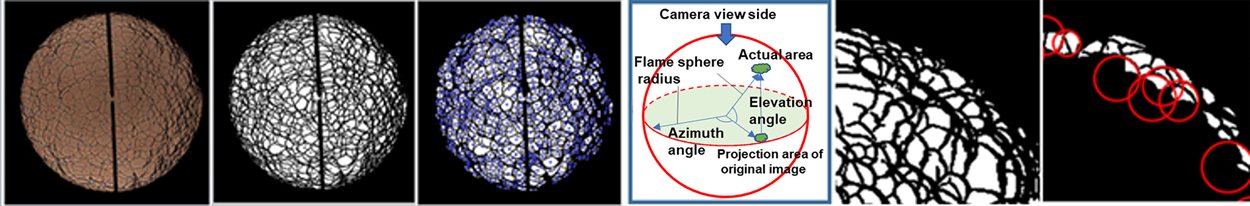
Figure 3. Image processing for quantitative information on cellular structures [Xu et al., 2017.03]
Combustion
Detailed combustion analysis has been conducted using DMF. The world’s first publication on DMF combustion in engines was made in Energy and Fuels, Impact Factor 2.72 (Zhong et al.). This used fixed spark timings to investigate the knock suppression ability of DMF compared to gasoline and ethanol. The effect of DMF on combustion using optimised spark timings and loads was then published in FUEL, Impact Factor 3.79 (Daniel et al. a). This was the world’s second publication on DMF as engine fuel. Since these two publications, several more journal papers and SAE papers have followed and these included the publication on the effect of various engine parameters, such as engine load, optimum spark timing, injection timing and valve timing when using DMF (Daniel et al. b) and the effect of split-injection at full load (Daniel et al. c). The engine performance and emissions are less sensitive to changes in key control parameters than when using DMF compared to gasoline. This was also shown using spark sensitivities (Daniel et al. a). This allows a wider window for improving performance and/or reducing emissions. Novel fuel preparation techniques have been investigated by comparing traditional externally supplied gasoline-biofuel blends to internally mixed, dual-injection blends. The results using low blends of DMF with gasoline (25% of DMF by volume, or D25) are more efficiently utilised than in external blends (Daniel et al. d).
Pure MF was tested at the optimum spark timing and the results were compared with DMF and gasoline. It was found that both MF and DMF have improved knock suppression over gasoline and the MF produces the highest indicated thermal efficiency, which is attributed to the faster burning rate and reduced knock suppression seen from MF (Wang et al.). Additionally, MF tests resulted in almost 30% lower volumetric indicated specific fuel consumption compared with ethanol. Addition of limited amounts of MF to gasoline showed negligible changes in combustion characteristics, however led to an increase in the combustion stability with a shorter combustion duration, MFB10 being 0.5-1 CAD shorter over load ranging from 3.5 – 8.5 bar BMEP.
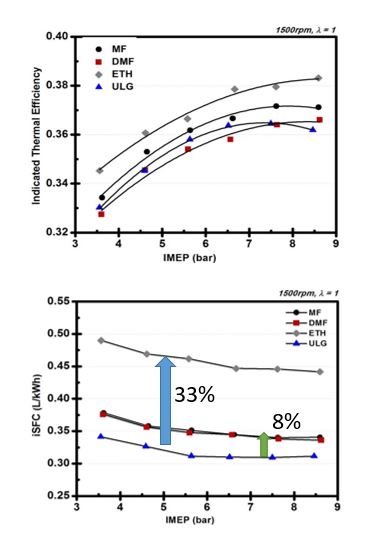
Figure 4. Thermal efficiency and fuel consumption (GDI engine, 1500 rpm, λ = 1.0, Knock Limited Spark Advance)
Regulated and unregulated emissions
CO2 emissions using dual-injection were shown to be similar to homogenous DI when using gasoline (Daniel et al.). However, the NOx emissions are much higher with DMF due to higher combustion pressure and temperatures. Furthermore, the particulate matter (PM) emissions can be reduced with dual-injection because gasoline is supplied through PFI. In terms of unregulated emissions, the emissions of the major carbonyls are lower when using DMF compared to gasoline and even less so than ethanol, which heavily emits acetaldehyde and formaldehyde. The formation of benzene and toluene during DMF combustion results in similar benzaldehyde emissions to gasoline. The HC emissions of DMF are dominated by unburned fuel.
HC emissions in case of MF were found to reduce while NOx stayed similar to the pure gasoline cases. Overall regulated emissions (CO, NOx and HC) and particulate matter (PM) were typically similar as gasoline (Wang et al.). The overall consensus of unregulated emissions was that MF produced a lower aldehyde emissions in comparison to gasoline and bio-ethanol (Wang et al.).
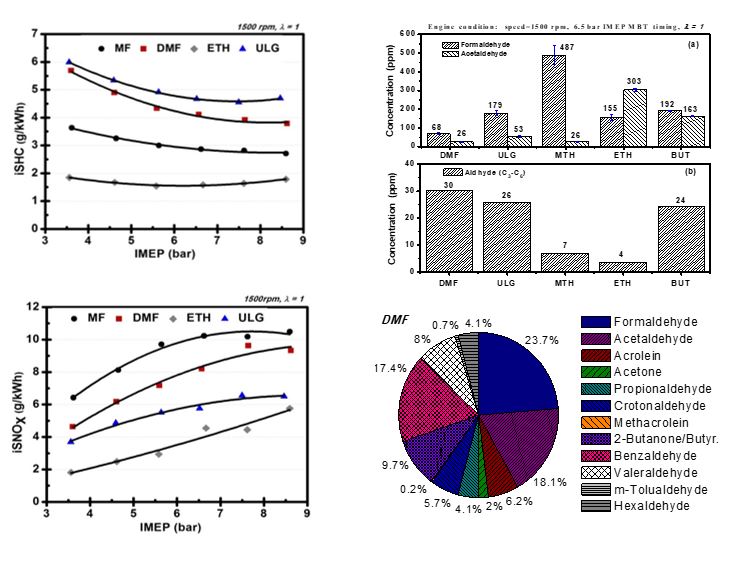
Figure 5. Emissions (GDI 1500 rpm, λ=1.0, Knock Limited Spark Advance)
Impact to engine hardware and environment
The lubricity of DMF was jointly studied at the Hefei University of Technology in China which revealed a more favourable lubricity of DMF than gasoline (Hu et al. 2012). The toxicity of 2,5-Dimethylfuran (DMF) was investigated by a team in the School of Biosciences. Compared to the sub-toxic atmospheric concentration of gasoline of around 0.6 mM, DMF is shown to be sub-toxic at intracellular concentrations up to 5 mM in human liver cells. This appears promising because intracellular concentrations will be much lower than those observed in the atmosphere due to the distribution of the compound (Whyley et al.).
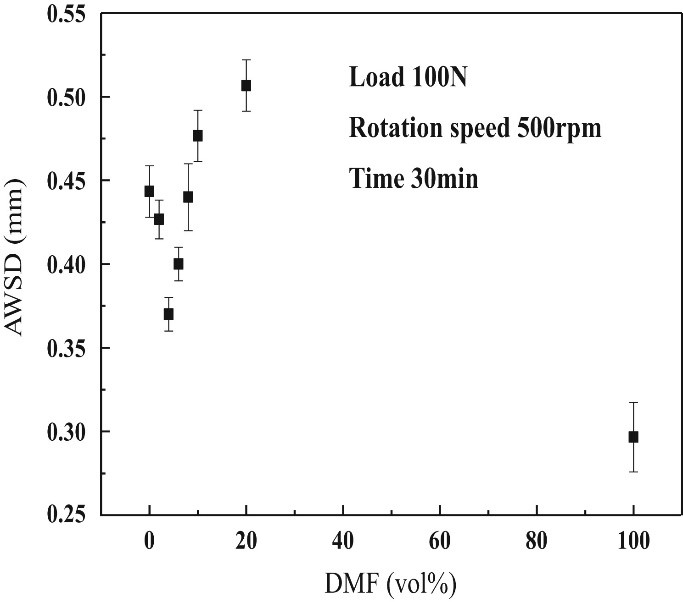
Figure 6. Average wear scar diameter (AWSD) variation with DMF blending into gasoline
Project report date:
March 2017