 This is to briefly tell you about the MEDAL trial that is running in this GP practice. If, after reading this, you would like to know more or take part, please read the full participant information sheet: Patient Information Sheet v3.0 (14-Jan-2025)
This is to briefly tell you about the MEDAL trial that is running in this GP practice. If, after reading this, you would like to know more or take part, please read the full participant information sheet: Patient Information Sheet v3.0 (14-Jan-2025)
What is the MEDAL trial trying to find out and who are we?
MEDAL trial aims to find out which medications, either on their own, or in combination with others, give the best relief from acute back pain. It is the first of its kind. You will be helping the MEDAL team find out the best medications to treat low back pain which will help tackle one of the biggest health problems in the UK and reduce suffering for people who get low back pain in the future. It is a national trial and is run by the Birmingham Clinical Trials Unit (BCTU) at the University of Birmingham. The University of Birmingham is the Sponsor of the trial and BCTU is responsible for the day to day running of the trial. The trial is funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme.
Why have I been asked to take part in the trial?
You have been asked to take part because you are an adult and currently experiencing low back pain with or without related leg pain, or sciatica that you have had for less than 3 months.
Will I get paid to take part?
You will not be paid to take part in the trial. However, you will be offered a £10 gift voucher, after attending the baseline appointment with the nurse and a £15 gift voucher after the 8 weeks follow up is complete, to say thank you for your time.
What happens in the trial?
- On calling your GP practice to book an appointment to discuss your back pain, they will believe that this trial might be appropriate for you and will send you a link to this information sheet. Alternatively, you may have seen a poster advertising this trial and visited our website.
- After reading patient information sheet and watching the summary video, we will need you to complete a self-assessment questionnaire of your back pain by clicking the link on the website given to you. Before completing the form, you will be asked to confirm that you have read the patient information sheet and agree to complete the form. The form will take no more than 10 minutes to complete, and your answers will help us to understand if this trial is right for you. If it is, you will be contacted by a research nurse who will discuss things further and will ask you to complete our consent process.
- The nurse will help you to download the MEDAL trial App. This is where participants will access their daily and follow up questionnaires.
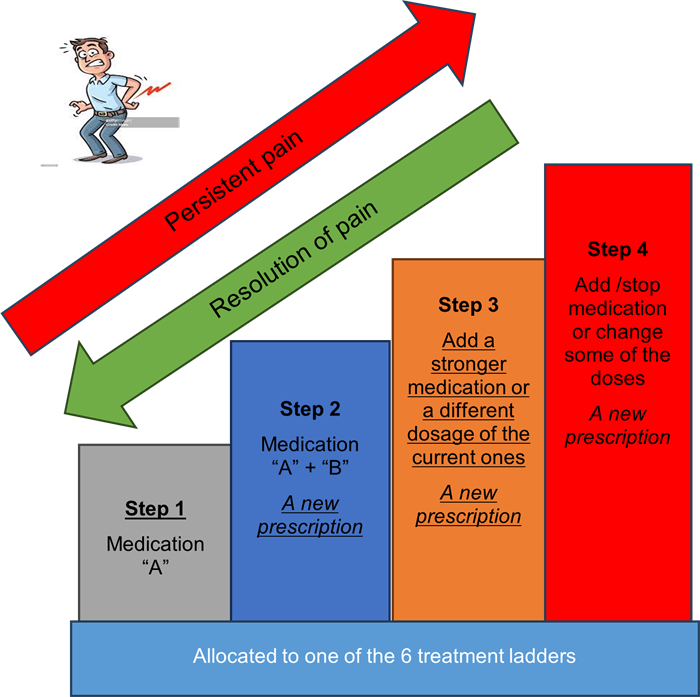
- After completing the initial assessment and consented to participate you will then have the GP appointment that you booked when you initially called your GP practice. The GP will confirm your eligibility to take part in the MEDAL trial and will randomly allocate you to one of six treatment plans or “ladders” as we call them in this trial. More details on the treatment ladder can be found on the participant information sheet.
- The GP will then issue a prescription for the first step of your treatment plan and give you the instructions for escalating/de-escalating your medications depending on your pain level.
- If, after a minimum of 3 whole days, you feel there hasn’t been enough improvement in your pain, you will be able to contact your GP practice, via their repeat prescription process, to collect the next medication on your treatment plan. This process can be repeated if your pain does not go away, until you are on the last step of your treatment plan.
- As your pain improves, you will be able to reduce the amount of medication you are taking.
Do I have to take part?
No, this is entirely up to you. Taking part is voluntary. You do not have to take part if you do not want to. It will not affect your standard of care in any way.
What can I do next?
If you think you might be interested in taking part, please take some time to read the full participant information sheet.
Download MEDAL Summary Participant Information Sheet